Latest News Feed
Consumer Safety Watch answers the following questions: Is it too late to qualify? Is there a deadline? What are the criteria to be part of the settlement?


Often taken long term for heartburn, ulcers, GERD, acid reflux, these drugs were only intended for short term use and are highly addictive.

The vehicles involved in the recall include the 2010 Chrysler Sebring midsize car, 2011 to 2014 Chrysler 200 midsize cars and the 2010 to 2012 Dodge Caliber compacts, Dodge Avenger midsize and Jeep Patriot and Compass vehicles.

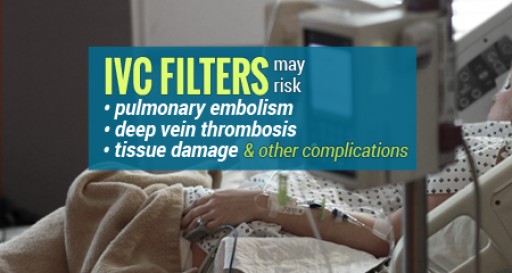
Many recipients of retrievable IVC filters, designed to protect against pulmonary embolisms, may have complications and yet not even be aware that there is a danger associated with the devices being left in place past a specified period of time. Devices left in place longer than 50 days may increase the risk of blood clots and deep vein thrombosis as well as other complications that include migration, fracture, embolization, perforation and the inability to remove the devices.

Individuals who have suffered complications or life threatening injuries as a result of defective IVC filters have many legal options. Consumer Safety Watch offers free legal assistance and can help place injured victims and their families with qualified law firms that will accept their cases at no cost unless there is a favorable verdict or settlement.

According to Consumer Safety Watch, many families with children that suffered birth defects that include heart defects or cleft lip or palate were under the impression that they would not be able to recover compensation for injuries or medical bills due to statute of limitation laws. In reality, since there has never been an acknowledgment by GlaxoSmithKline of a connection between the use of their anti nausea drug and these defects, statute of limitations laws should not apply

Retrievable IVC Filters meant to be a temporary solution to pulmonary embolism risks in many situation do not get removed or become impossible to remove leading to additional serious complications, according to the IVC Filter Legal Help Center.

If not removed within 30-45 days, a dangerously high rate of so-called retrievable IVC filters, generally implanted to prevent pulmonary embolisms, experience complications that can lead to additional medical procedures, serious injury and death, and the risk generally outweighs any benefit, according to Ford and Associates Nationwide Legal Services.

The controversial devices are linked to at least 27 deaths and over 300 additional injuries. Many devices can become difficult or impossible to retrieve if left in for more than 30-45 days after implant.

Recent studies suggest that use of the popular new age drugs known as SGLT2 Inhibitors used to treat type 2 diabetes show links to deadly side effects including DKA or diabetic ketoacidosis, kidney failure, heart attack and stroke.
A recent three part NBC News Investigation has raised serious concerns regarding the safety and FDA Approval Process of some IVC Filters.

For individuals suffering from symptoms mimicking a brain tumor, such as headaches, nausea, vomiting and vision loss, with no signs of an actual tumor, it could be connected to Mirena IUD, Ortho Evra or Depo Provera or another type of hormonal birth control causing a rare but serious condition called pseudotumor cerebri, which occurs when the pressure inside your skull increases for no obvious reason.
The makers of inferior vena cava (IVC) filters like Cook Medical's Celect and Gunther Tulip filters and C.R. Bard's Recovery and G2 filters face a growing number of product liability lawsuits brought on behalf of patients across the country who were implanted with the filters to reduce their risk of blood clots and pulmonary embolism, and suffered severe complications like filter migration, perforation of the vena cava and filter embolization.

Transvaginal mesh has been plagued by reports of devastating injuries among women who received the implants to treat pelvic organ prolapse or stress urinary incontinence, and to date, close to 80,000 lawsuits have been consolidated in six multidistrict litigations against mesh manufacturers like C.R. Bard, Coloplast, Ethicon, Boston Scientific and American Medical Systems.
A new product liability lawsuit brought on behalf of a Mississippi man alleges that side effects of the Bard Eclipse inferior vena cava (IVC) filter caused him to experience serious injuries when the filter tilted out of position and perforated his vena cava.
As many as 90% of pregnant women experience some nausea during the early stages of pregnancy, and a small percentage of those women will experience vomiting during pregnancy that is so excessive it may result in dehydration, weight loss and other complications.

Individuals who use certain diabetes medications including Invokana, Farxiga, Invokamet and Jardiance should be mindful of these diabetic ketoacidosis symptoms.

Invokana Lawsuit Help Center is reviewing cases for injuries including diabetic ketoacidos, kidney failure, heart attack and stroke at no upfront cost to the injured party and no cost ever unless there is a favorable settlement or verdict

Injuries from Invokana and other diabetes medications are resulting in lawsuits being filed at an increasing rate

Individuals that have suffered injuries after using these medications may be eligible for compensation, says the Invokana Legal Help Center

Families with Children Born Before 2006 May Still Be Entitled to Compensation for Zofran Birth Defects

According to a recent NBC News Investigation on the dangers associated with certain IVC filters, Bard may have even hired a public relations firm to help with a cover up while delaying pulling the dangerous devices from the market.

NBC Investigation alleges serious danger and potential fraud with some IVC filter medical devices.

Birth control allegedly contributes to the likelihood of acquiring the injury also commonly called idiopathic intracranial hypertension. Excess fluid in the brain can contribute to severe headaches, blurry vision or double vision, permanent blindness and other injuries.

The popular anti-nausea medication, Zofran (ondansetron) is commonly prescribed to combat nausea and vomiting in patients undergoing surgery or chemotherapy treatment. However, Zofran is also sometimes prescribed off-label to pregnant women, as a treatment for a severe form of morning sickness called hyperemesis gravidarum.

Federal regulators are warning consumers and the medical community that a potentially life-threatening condition called diabetic ketoacidosis may be linked to a popular class of diabetes drugs known as SGLT2 (sodium-glucose cotransporter-2) inhibitors.

